Clinical Data Capture for Parkinson's Research
"Can your system help us collect data for a clinical trial?" Of course.
What started as way to help a longtime partner collect data for a small, yet important, interventional trial to treat epilepsy turned into the cornerstone for Blackfynn's strategic goals to improve therapeutic drugs through better data.
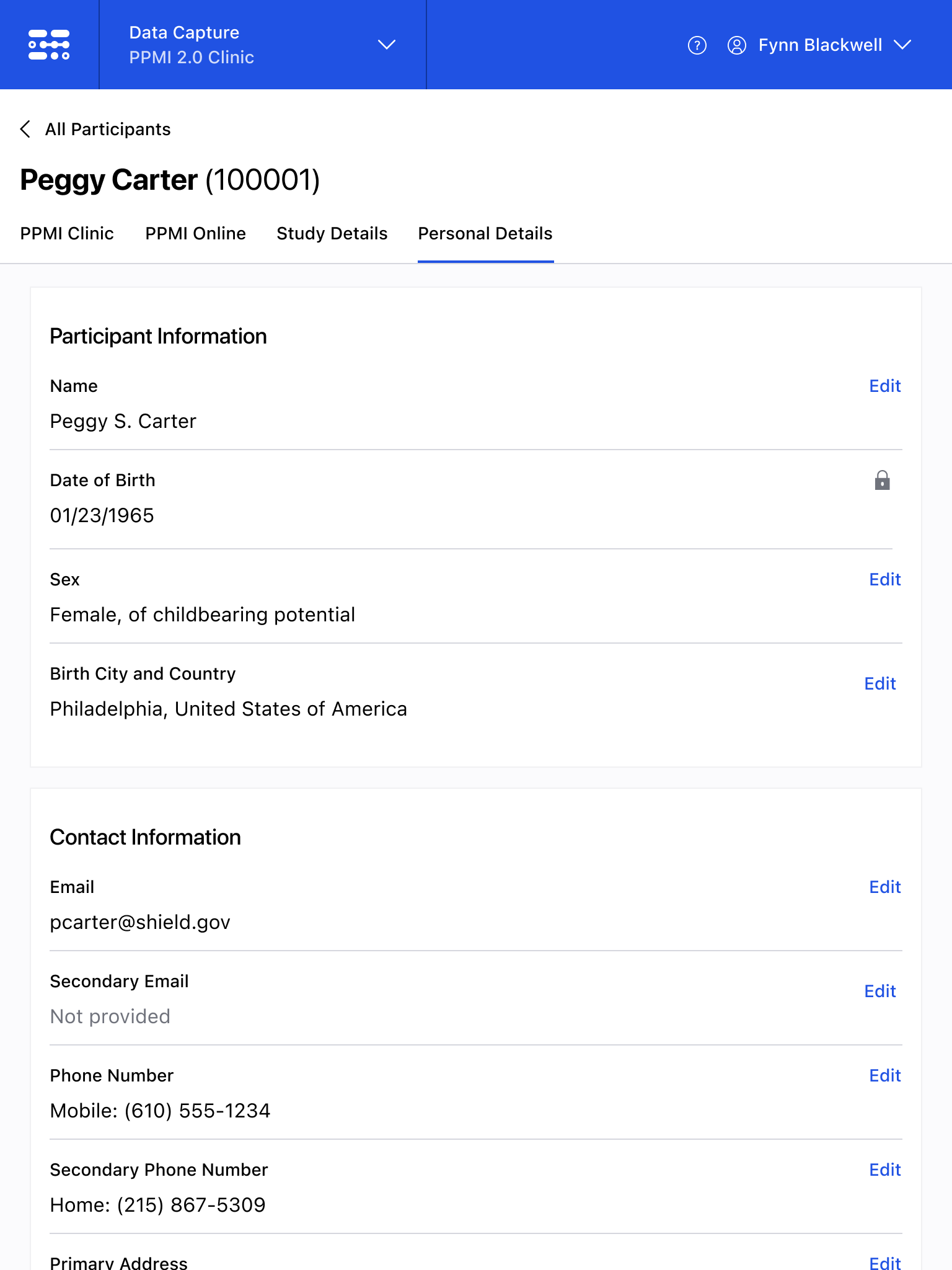
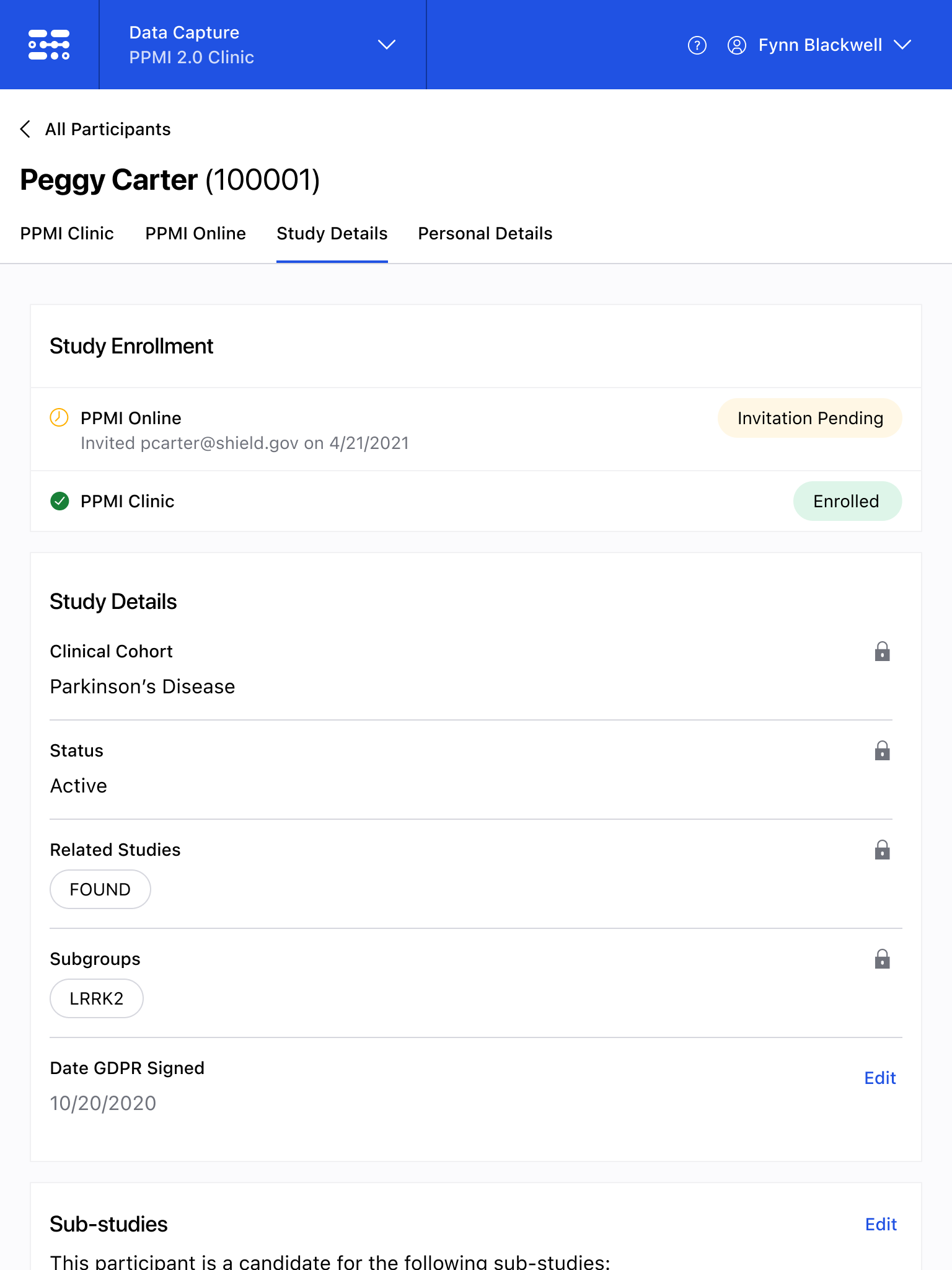
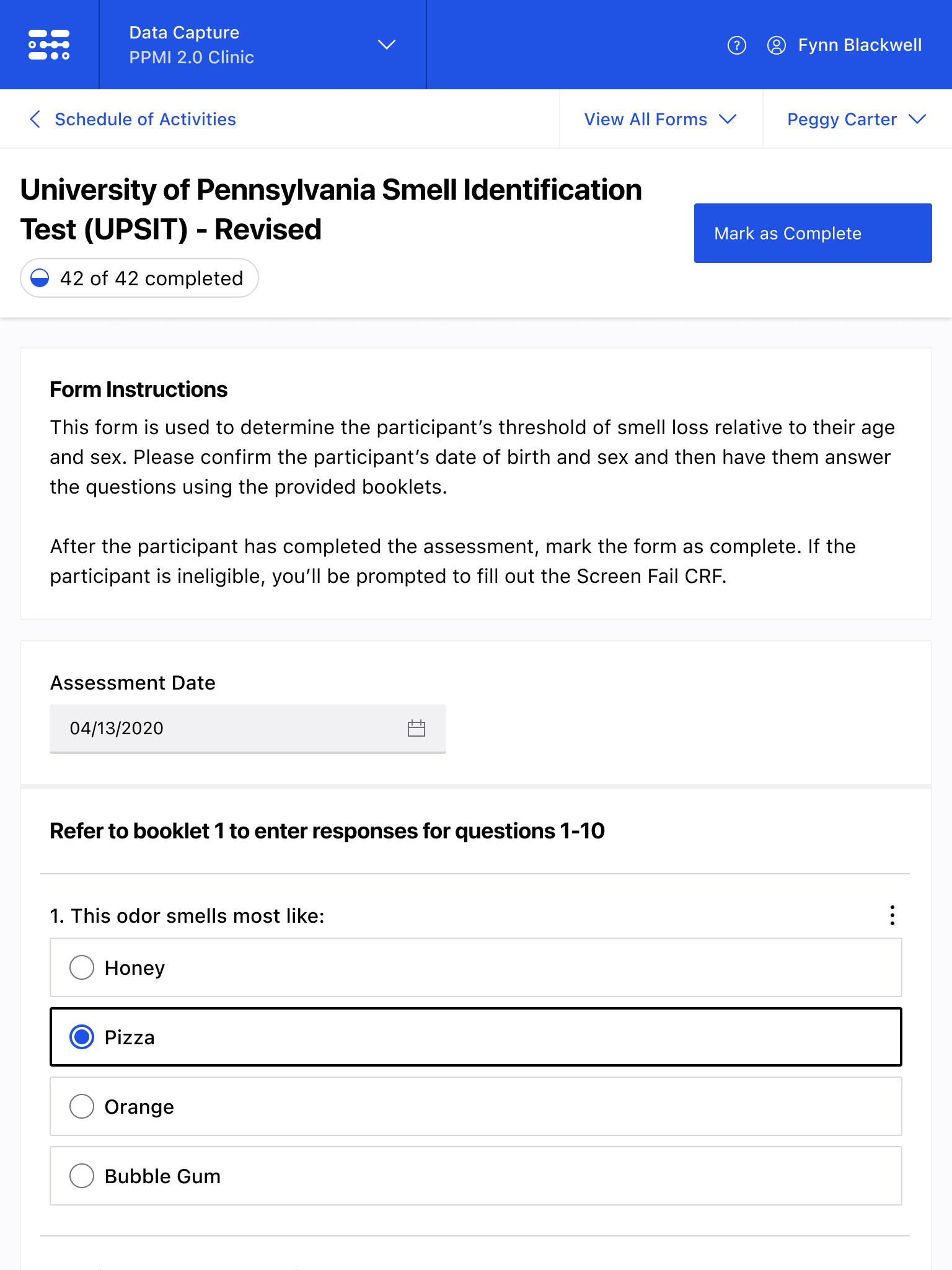
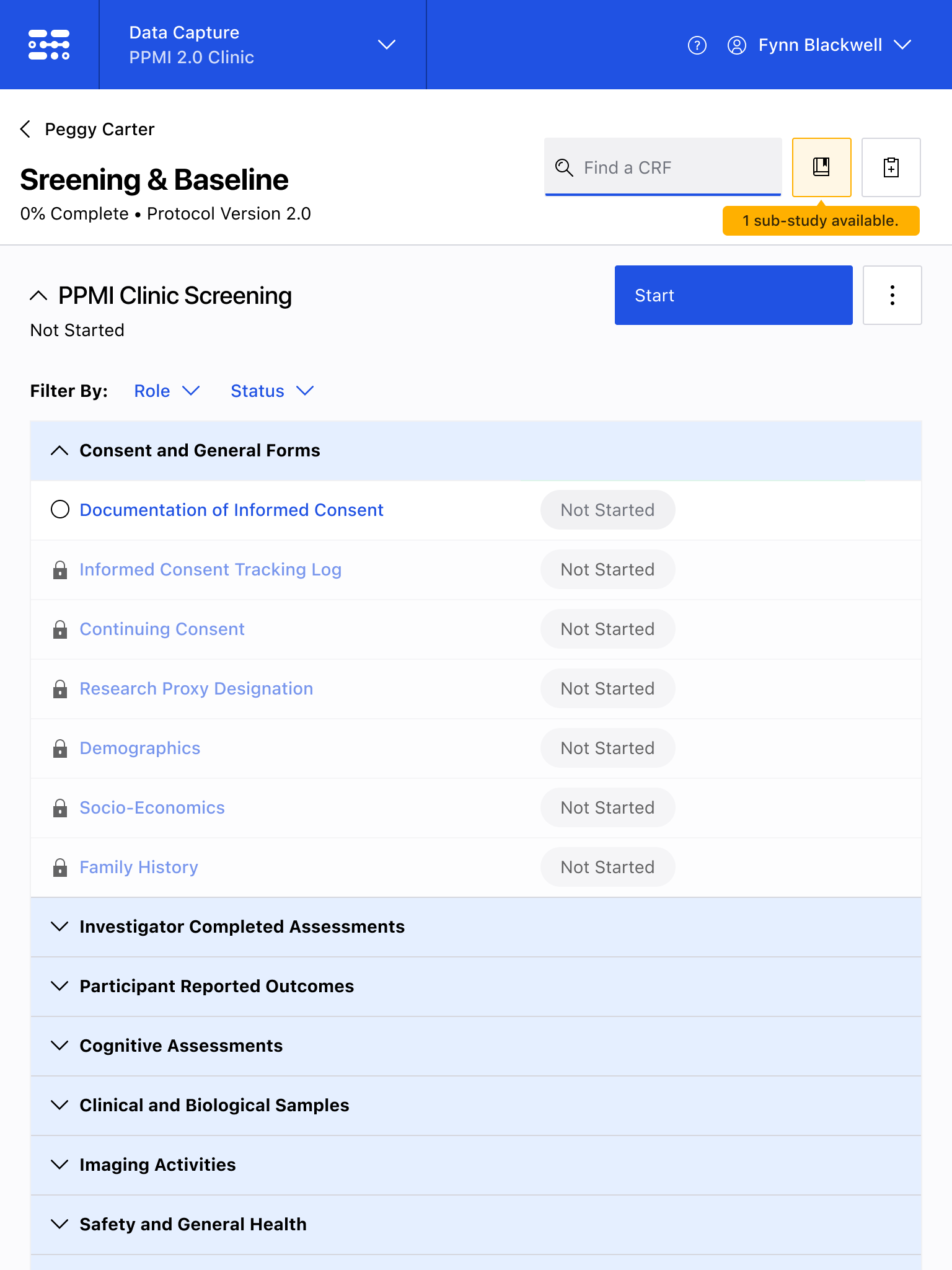
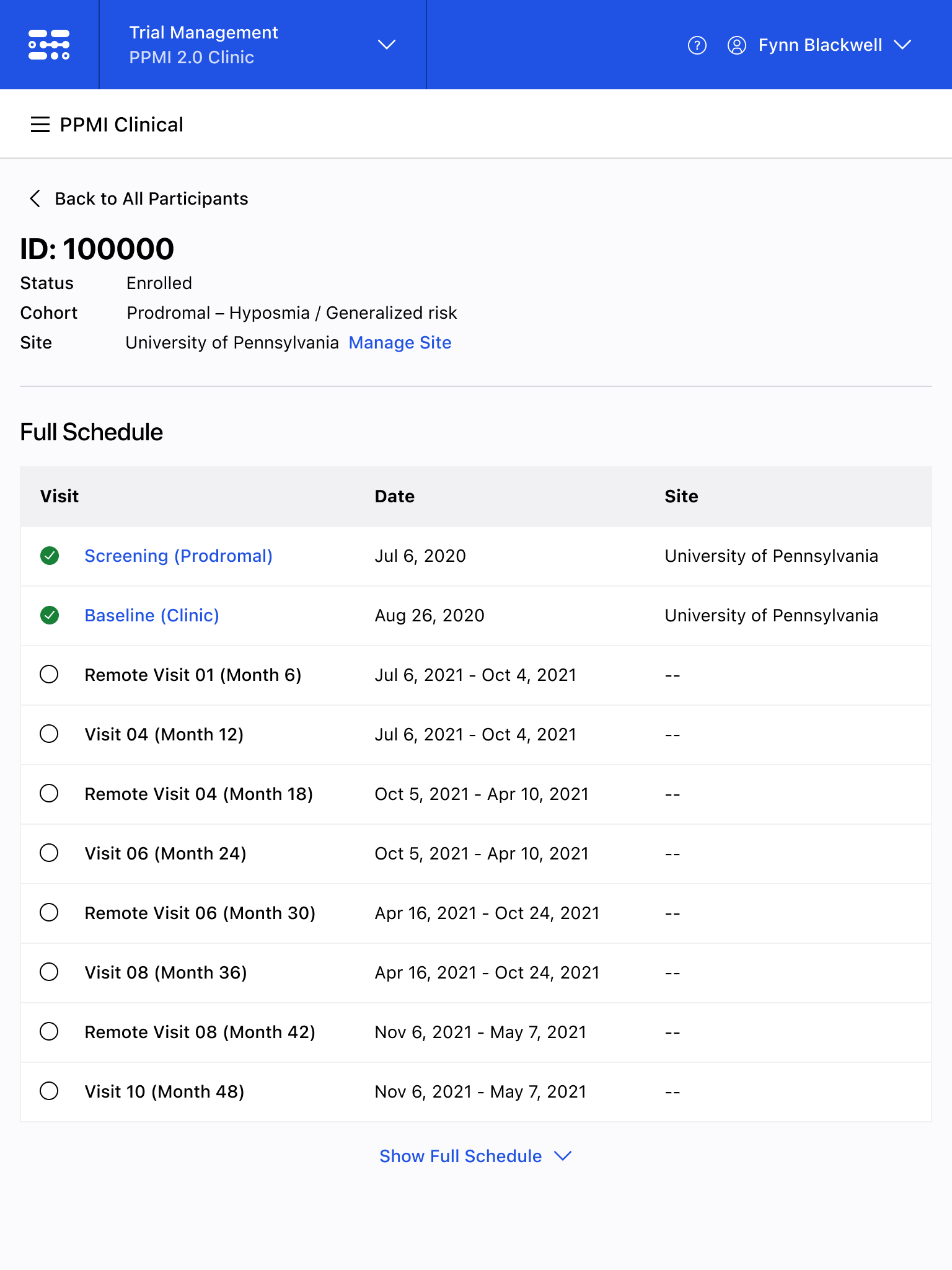
The Blackfynn Clinical Data Management System is a boutique Electronic Data Capture (EDC) and trial management platform optimized to support clinical trials targeting the neurosciences. The responsive, tablet-first experience was built from the ground up to eliminate confusion during the data collection process so that clinical staff could conduct visits as quickly as possible. Our goal was to enable clinical staff to easily capture clinical data, so that patients could get back to their lives. With some clinical trial visits taking six to eight hours to complete, it was important that we reduced friction to save as much time as possible.
I had the opportunity to contribute to the strategy of the platform from the beginning. Working with engineering and business leadership, I led the research and design effort to rapidly prototype and test what would later become the foundation for the CDMS platform. Leading a small team of designers through the early stages of the process eventually led me to create the Product Experience team that redefined how we developed our products at Blackfynn.
The end result was a lightweight clinical data capture platform that allowed the Michael J. Fox Foundation's Parkinsons Progression Markers Initiative (PPMI), to move away from a paper-based clinical assessments to direct data capture via iPads in the clinic. This resulted in real-time access to data and improved collection rates among sites.